Canhelp®-UCa CE-IVD
Diagnostic Test for Urothelial Cancer Detection
Utilità di Canhelp-UCa
Current guidelines for urothelial carcinoma support a diagnostic approach based on cystoscopy combined with sample urinary cytology.
There cystoscopy It's a procedure invasive, uncomfortable, which carries a high cost and risks of infection and trauma, but which remains the gold standard for the diagnosis of urinary tumors.
To limit its use, Canhelp Genomics has developed Canhelp-UCa, a diagnostic test with high sensitivity and specificity che migliora le percentuali di rilevamento del cancro della vescica. Queste caratteristiche rendono Canhelp-UCa uno strumento utile per ridurre l’utilizzo della cistoscopia, offrendo un metodo di screening più accessibile e meno invasivo.
Canhelp-UCa è un test diagnostico multigenico che permette di aiutare nella diagnosi e nel monitoraggio dei tumori uroteliali.
The test evaluates, starting from urine samples, the expression of 8 genes (CA9, CCL18, ERBB2, IGF2, MMP12, PPP1R14D, SGK2 and SWINGN) in an RT-PCR assay. The data is processed by software that uses artificial intelligence.
L’utilizzo di Canhelp-UCa porta a diversi benefici.
Benefits for the patient:
- The test is non-invasive and painless compared to cystoscopy
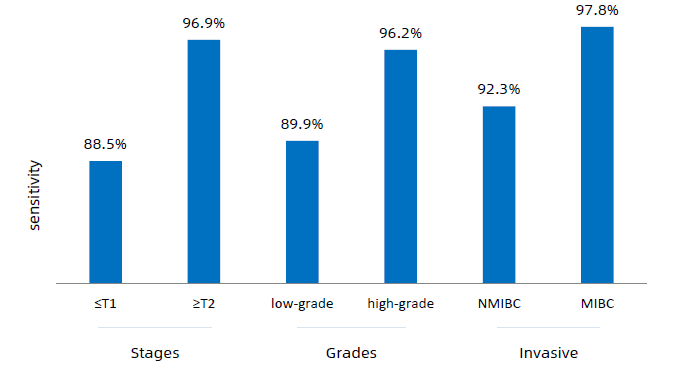
- increases the detection rate of bladder cancer in early and low-grade stages, improving prognosis
- reduces anxiety for patients who require frequent testing
Benefits for the healthcare facility:
- The test helps doctors obtain additional information for more accurate diagnosis and treatment
- increases the willingness of patients to undergo frequent examinations to monitor recurrences after surgery
- The test is easy to implement
Canhelp-UCa è un kit certificato CE IVD
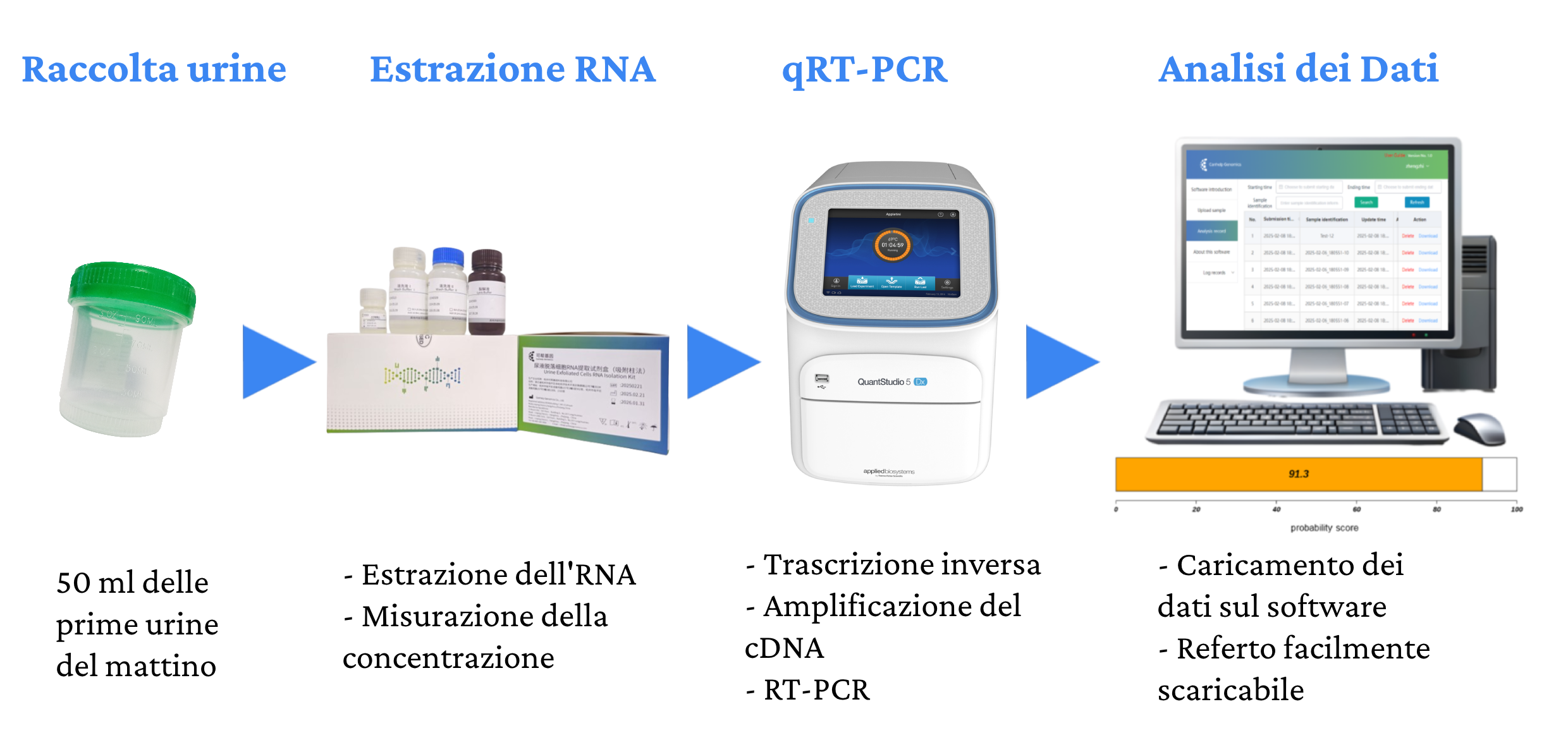
Canhelp-UCa si esegue direttamente in laboratorio su 50 ml delle prime urine del giorno.
Il test analizza tramite RT-PCR l’espressione di 8 geni dall’RNA estratto dal campione di urina e calcola il punteggio che classifica il risultato come positivo o negativo per la presenza del tumore. Il test Canhelp-UCa ha una elevata specificità e sensibilità per tutti i tumori, indipendentemente dallo stadio, con un’accuratezza del 93,4%, una sensibilità del 92,3% e una specificità del 94,1%.
Ogni kit Canhelp-UCa contiene il materiale e i reagenti per 24 test.
Procedure
La procedura per eseguire il test Canhelp-UCa prevede i seguenti passaggi:
Il referto di Canhelp-UCa
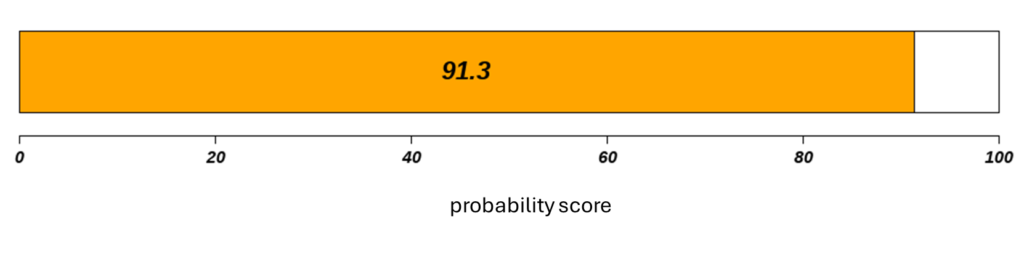
Il referto fornito da Canhelp-UCa include un punteggio di probabilità basato sull’analisi dell’espressione di specifici marker genetici nelle urine, offrendo informazioni utili per la diagnosi precoce e il monitoraggio delle recidive del cancro alla vescica. Grazie all’integrazione con software di intelligenza artificiale, il referto fornisce valutazioni tempestive, supportando le decisioni cliniche e contribuendo a ottimizzare la gestione del paziente.
If the probability score is:
- equal to or greater than 50: the result is to be considered positive
- less than 50: the result is to be considered negative
Bibliography
For more information click HERE
For information
Telephone
+39 0239261913
info@quimark.com
The content of this website is informative about the Quimark company and its products and services,
It is not intended as professional medical or health advice.