MyChoice®CDx PLUS


HRD test on tumor tissue
Determination of homologous recombination deficiency (HRD) in ovarian cancer
MyChoice CDx Plus is a genomic test that guides medical decisions in the treatment of ovarian cancer with PARP-inhibitor drugs.
The test is performed in laboratories Myriad Genetics USA on a sample of ovarian tumor tissue.
Mutations are searched for BRCA1 and BRCA2 and the genomic instability score is determined GIS (Genomic Instability Score), to obtain an integrated and accurate HRD score. (1-4).
HRD = BRCA1/2+GIS
MyChoice CDx Plus promptly identifies patients eligible for drug treatment PARP inhibitors and is the most validated HRD test in ovarian cancer. (1,5-8)
MyChoice CDx Plus is the only HRD test with
-
- FDA approval,(9)
- certification THERE IS,
- recommendation in the main international guidelines on the treatment(10-12) and
- prospective data from phase III studies.(1,5-8)
MyChoice CDx Plus is a CE marked device, designed to comply with applicable standards and general safety and performance requirements of the European Directive 98/79/CE (in vitro diagnostic medical devices). The declaration of conformity is issued under the responsibility of Myriad Genetic Laboratories, Inc.
Bibliography
1. Ray-Coquard, Isabelle, et al. “Olaparib Plus Bevacizumab as First-Line Maintenance in Ovarian Cancer.” New England Journal of Medicine, vol. 381, no. 25, 2019, pp. 2416–2428.
2. Timms, Kirsten M, et al. “Comparison of Genomic Instability Test Scores Used for Predicting PARP Activity in Ovarian Cancer.” Journal of Clinical Oncology, vol. 38, no. 15_suppl, 2020, pp. 1586–1586.
3. Watkins, Johnathan A et al. “Genomic Scars As Biomarkers Of Homologous Recombination Deficiency And Drug Response In Breast And Ovarian Cancers”. Breast Cancer Research, vol 16, no. 3, 2014.
4. The Cancer Genome Atlas Research Network. Nature 2011.
5. Ray-Coquard, Isabelle, et al. “Olaparib Plus Bevacizuamb First-Line Maintenance in Ovarian Cancer: Final Overall Survival Results from the Paola-1/ENGOT-OV25 Trial.” Annals of Oncology, vol. 34, no. 8, 2023, pp. 681–692.
6. González-Martín, Antonio, et al. “Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer.” New England Journal of Medicine, vol. 381, no. 25, 2019, pp. 2391–2402.
7. González-Martín, Antonio, et al. “Progression-Free Survival and Safety at 3.5 Years of Follow-up: Results from the Randomized Phase 3 Prima/Engot-OV26/GOG-3012 Trial of Niraparib Maintenance Treatment in Patients with Newly Diagnosed Ovarian Cancer.” European Journal of Cancer, vol. 189, 2023, p. 112908.
8. Coleman, Robert L., et al. “Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer.” New England Journal of Medicine, vol. 381, no. 25, 2019, pp. 2403–2415.
9. Cristescu, Razvan et al. “428 Genomic Instability Metric Concordance Between Oncoscan™, Cytosnp And An FDA-Approved HRD Test”. Translational Research, 2020.
10. Tew, William P., et al. “Poly(ADP-Ribose) Polymerase Inhibitors in the Management of Ovarian Cancer: ASCO Guideline Rapid Recommendation Update.” Journal of Clinical Oncology, vol. 40, no. 33, 2022, pp. 3878–3881.
11. Miller, Rowan E. et al. “ESMO Recommendations On Predictive Biomarker Testing For Homologous Recombination Deficiency And PARP Inhibitor Benefit In Ovarian Cancer”. Annals Of Oncology, vol 31, no. 12, 2020, pp. 1606-1622.
12. National Comprehensive Cancer Network. Ovarian Cancer/Fallopian Tube Cancer/Primary Peritoneal Cancer. Version 1.2023.
Knowing your HRD status is critical to determining ovarian cancer treatment options
A condition of homologous recombination deficiency (HRD) is present in the 48% about ovarian tumors(1) and is Often due to a mutation that is found only in cancer.

However, while some HRD causes are well established, others remain unknown.(2,3,4)
Determining HRD status for ovarian cancer patients may help provide insight into the extent of benefit from therapy with PARP inhibitors, which determines optimal outcomes for patients with HRD+.
The Technical specifications of the MyChoice CDx Plus test are found here.
The evaluation of LOH, TAI and LST allows the determination of HRD regardless of the specific cause.(3), while it would be very difficult to examine each individual cause. This is why_
● HRD resulting from epigenetic events such as BRCA1 promoter methylation would not be detected with a gene sequencing approach.(5,6)
● HR gene mutations other than BRCA1 and BRCA2 are rare and it is unclear whether they are linked to HRD.(7,8)
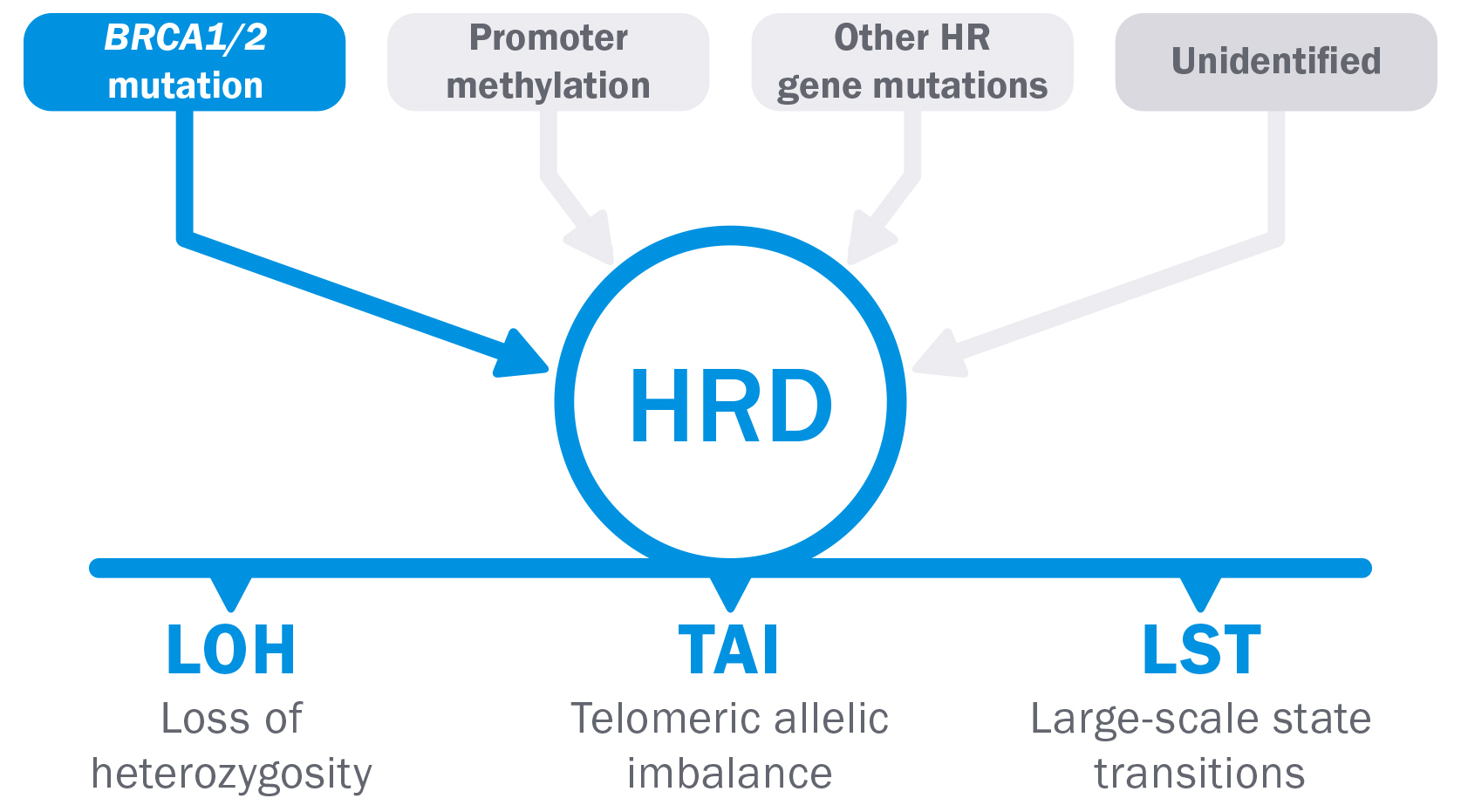
Unidentified causes of HRD are not detected.
 |
BRCA1/2
|
 |
LOH + TAI + LST
|
MyChoiceCDx PLUS determines HRD status in various ways, for more comprehensive results.
MyChoice screens ovarian cancer tumors using two methods (BRCA1/2 mutation and genomic instability) to determine a patient's HRD status.
MyChoice, the benchmark HRD test
Accurate
MyChoice identifies 34% in more HRD tumors than tests using %LOH alone.5
Quick
Doctors will receive a MyChoice result within 2-3 weeks of the laboratory receiving the sample.
Operable
Easy-to-read MyChoice results with GIS interpretation, BRCA1/BRCA2 and combined results to make informed treatment decisions
Reliable
FDA, PMDA, ASCO, NCCN and ESMO trust MyChoice. (9-12)
The most complete and accurate HRD test to make therapeutic decisions in a few days
MyChoice has been shown to identify 34% in more HRD tumors than other methods that use %LOH alone (5), providing more accurate information to make informed treatment decisions.
● 27,000 SNPs allow for a more refined analysis of the genome than %LOH alone, which uses 3,500 SNPs and examines only a portion of the genome.
● Technology that analyzes BRCA1/2 for sequence variants and large rearrangements, thus capturing more 5% than other platforms that do not have this technology.5,13,14
Full clinical validation
MyChoice is the only HRD test prospectively validated in three phase III studies for use in the first-line treatment of ovarian cancer.14-18
Robust long-term data confirm that the MyChoice test improves survival in patients with newly diagnosed advanced ovarian cancer receiving first-line treatments.14-18
PAOLA-1 n= 806
FIRST n= 733
VELIA n=1.140
HRD tests are not interchangeable
| Other HRD tests |
MyChoiceCDx
|
||
| BRCA1 & BRCA2 | Sequence Variants | Yes | Yes |
|
Extensive Rearrangements
|
No | Yes | |
| Genomic Instability Score | Loss of heterozygosity (LOH) | Yes | Yes |
|
Telomeric allelic imbalance (TAI)
|
No | Yes | |
| Large-scale state transitions (LST) | No | Yes |
Trusted around the world
MyChoice CDx is mentioned in the guidelines and is the only HRD test with Level of Evidence 1A (LoE1A) validated in several phase III studies for the first-line treatment of ovarian cancer with PARP inhibitors.14-18
Recommended by NCCN(12)
Somatic testing should prioritize the identification of molecular alterations that allow for effective interventions. This includes assessment of BRCA1/2 status, loss of heterozygosity (LOH), or homologous recombination deficiency (HRD) in the absence of a germline BRCA mutation.
Recommended by ASCO(9)
Myriad MyChoice CDx is included in the American Society of Clinical Oncology (ASCO) recommendations for the use of PARP inhibitors for the treatment and management of certain patients with advanced ovarian cancer.
Recommended by ESMO(10)
Validated HRD tests based on genomic scarring can be used to guide treatment with PARP inhibitors.
ESMO recognizes that MyChoice CDx is the only scar-based HRD test validated in the first-line maintenance setting.
Clear and understandable reports
Key elements of the report:
● Genomic Instability Score (GIS).
● Tumor BRCA1/BRCA2 mutation (tBRCA1/2)
● Interpretation of any somatic BRCA1 and BRCA2 variants
● Overall interpretation of the test most relevant for patient management (if multiple tests are detected) variants)
Technical Specifications (in English)
Bibliography
1. Moore, Kathleen N, et al. “Niraparib Monotherapy for Late-Line Treatment of Ovarian Cancer (Quadra): A Multicentre, Open-Label, Single-Arm, Phase 2 Trial.” The Lancet Oncology, vol. 20, no. 5, 2019, pp. 636–648.
2. Da Cunha Colombo Bonadio, Renata Rodrigues, et al. “Homologous Recombination Deficiency in Ovarian Cancer: A Review of Its Epidemiology and Management.” Clinics, 73(Suppl 1), 2018, p. e450s.
3. Watkins, Johnathan A et al. “Genomic Scars As Biomarkers Of Homologous Recombination Deficiency And Drug Response In Breast And Ovarian Cancers”. Breast Cancer Research, vol 16, no. 3, 2014.
4. Konstantinopoulos, Panagiotis A., et al. “Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer.” Cancer Discovery, vol. 5, no. 11, 2015, pp. 1137–1154.
5. Timms, Kirsten M, et al. “Comparison of Genomic Instability Test Scores Used for Predicting PARP Activity in Ovarian Cancer.” Journal of Clinical Oncology, vol. 38, no. 15_suppl, 2020, pp. 1586–1586.
6. Baldwin, Rae L. et al. “BRCA1 Promoter Region Hypermethylation in Ovarian Carcinoma: A Population-based Study.” Cancer Research, vol. 60, no. 19, 2000, pp. 5329-5333.
Norquist, Barbara M. et al. “Inherited Mutations In Women With Ovarian Carcinoma”. JAMA Oncology, vol 2, no. 4, 2016, p. 482.
7. The Cancer Genome Atlas Research Network. “Integrated Genomic Analyzes Of Ovarian Carcinoma”. Nature, vol 474, no. 7353, 2011, pp. 609-615.
8. Weichert, W et al. “216 An Evaluation Of The Performance Of Molecular Assays To Identify Homologous Recombination Deficiency-Positive Tumors In Ovarian Cancer”. Late Breaking Abstracts, 2021.
9. Tew, William P., et al. “Poly(ADP-Ribose) Polymerase Inhibitors in the Management of Ovarian Cancer: ASCO Guideline Rapid Recommendation Update.” Journal of Clinical Oncology, vol. 40, no. 33, 2022, pp. 3878–3881.
10. Miller, Rowan E. et al. “ESMO Recommendations On Predictive Biomarker Testing For Homologous Recombination Deficiency And PARP Inhibitor Benefit In Ovarian Cancer”. Annals Of Oncology, vol 31, no. 12, 2020, pp. 1606-1622.
11. Cristescu, Razvan et al. “428 Genomic Instability Metric Concordance Between Oncoscan™, Cytosnp And An FDA-Approved HRD Test”. Translational Research, 2020.
12. National Comprehensive Cancer Network. Ovarian Cancer/Fallopian Tube Cancer/Primary Peritoneal Cancer. Version 1.2023.
13. https://info.foundationmedicine.com/hubfs/FMILabels/FoundationOne_CDx_Label_Technical_Info.pdf
14. Ray-Coquard, Isabelle, et al. “Olaparib plus Bevacizumab First-Line Maintenance in Ovarian Cancer: Final Overall Survival Results from the Paola-1/ENGOT-OV25 Trial.” Annals of Oncology, vol. 34, no. 8, 2023, pp. 681–692.
15. Ray-Coquard, Isabelle, et al. “Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer.” New England Journal of Medicine, vol. 381, no. 25, 2019, pp. 2416–2428.
16. González-Martín, Antonio, et al. “Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer.” New England Journal of Medicine, vol. 381, no. 25, 2019, pp. 2391–2402.
17. González-Martín, Antonio, et al. “Progression-Free Survival and Safety at 3.5 Years of Follow-up: Results from the Randomized Phase 3 Prima/Engot-OV26/GOG-3012 Trial of Niraparib Maintenance Treatment in Patients with Newly Diagnosed Ovarian Cancer.” European Journal of Cancer, vol. 189, 2023, p. 112908.
18. Coleman, Robert L., et al. “Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer.” New England Journal of Medicine, vol. 381, no. 25, 2019, pp. 2403–2415.
19. Denkert, Carsten, et al. “Homologous Recombination Deficiency as an Ovarian Cancer Biomarker in a Real-World Cohort.” The Journal of Molecular Diagnostics, vol. 24, no. 12, 2022, pp. 1254-1263.
For information
Telephone
+39 0240090222
oncology@did.it
The content of this website is informative about the DID Spa company and its products and services,
It is not intended as professional medical or health advice.


