Canhelp®-UCa CE-IVD
Diagnostic Test for Urothelial Cancer Detection
Canhelp-UCa Utility
Current guidelines for urothelial carcinoma support a diagnostic approach based on cystoscopy combined with sample urinary cytology.
There cystoscopy It's a procedure invasive, uncomfortable, which carries a high cost and risks of infection and trauma, but which remains the gold standard for the diagnosis of urinary tumors.
To limit its use, Canhelp Genomics has developed Canhelp-UCa, a diagnostic test with high sensitivity and specificity that improves bladder cancer detection rates. These features make Canhelp-UCa a useful tool to reduce the use of cystoscopy, offering a more accessible and less invasive screening method.
Canhelp-UCa is a multigene diagnostic test that can aid in the diagnosis and monitoring of urothelial tumors.
The test evaluates, starting from urine samples, the expression of 8 genes (CA9, CCL18, ERBB2, IGF2, MMP12, PPP1R14D, SGK2 and SWINGN) in an RT-PCR assay. The data is processed by software that uses artificial intelligence.
Using Canhelp-UCa brings several benefits.
Benefits for the patient:
- The test is non-invasive and painless compared to cystoscopy
- increases the detection rate of bladder cancer in early and low-grade stages, improving prognosis
- reduces anxiety for patients who require frequent testing
Benefits for the healthcare facility:
- The test helps doctors obtain additional information for more accurate diagnosis and treatment
- increases the willingness of patients to undergo frequent examinations to monitor recurrences after surgery
- The test is easy to implement
Canhelp-UCa is a CE IVD certified kit
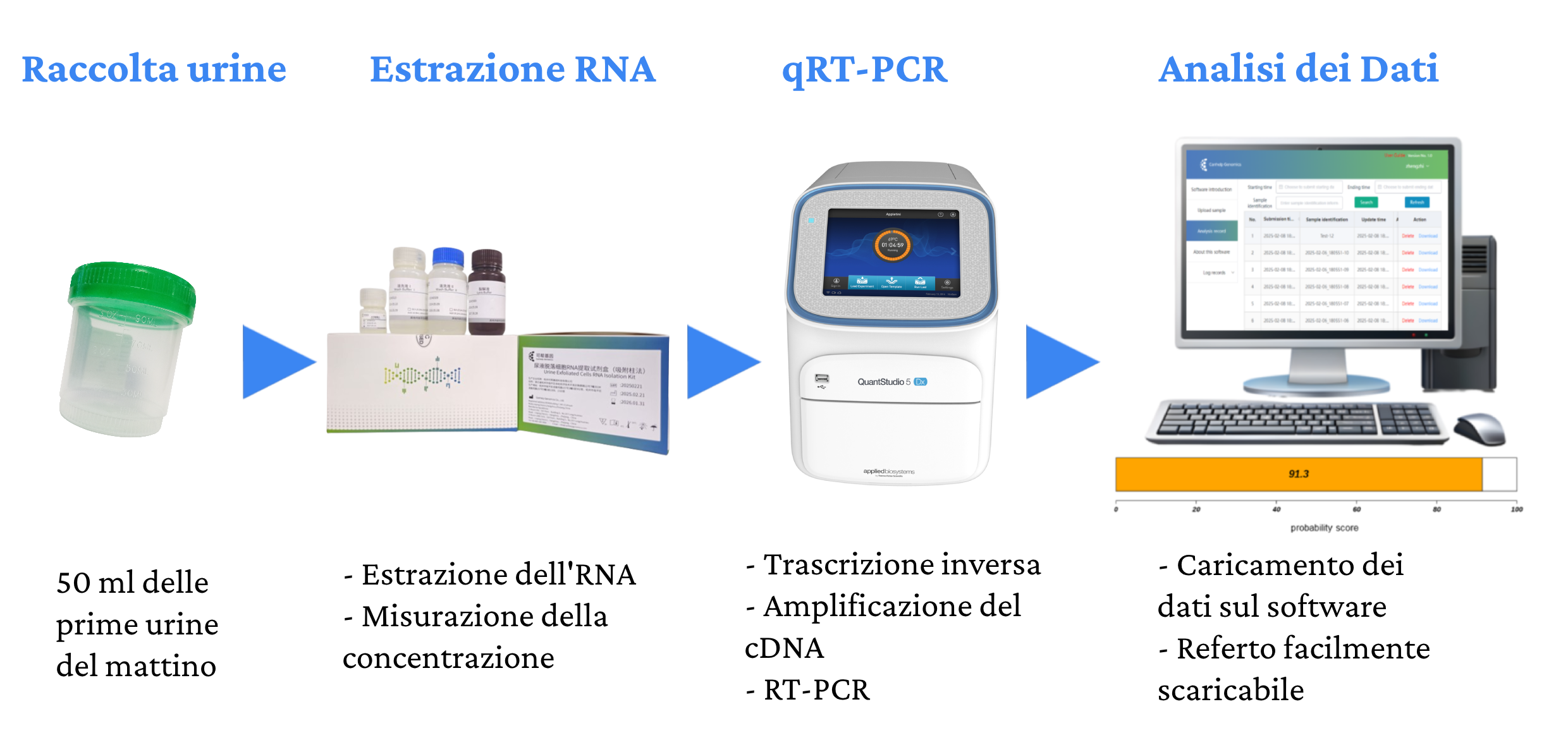
Canhelp-UCa is performed directly in the laboratory on 50 ml of the first urine of the day.
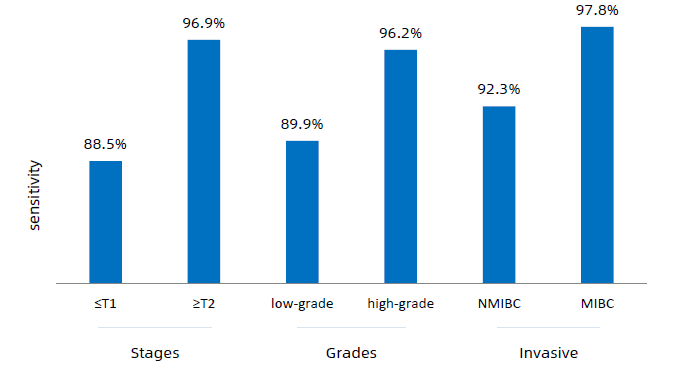
The test analyzes the expression of 8 genes from the RNA extracted from the urine sample by RT-PCR and calculates the score that classifies the result as positive or negative for the presence of the tumor. The Canhelp-UCa test has a high specificity and sensitivity for all tumors, regardless of stage, with an accuracy of 93.4%, a sensitivity of 92.3% and a specificity of 94.1%.
Each Canhelp-UCa kit contains materials and reagents for 24 tests.
Procedure
The procedure for performing the Canhelp-UCa test involves the following steps:
The Canhelp-UCa report
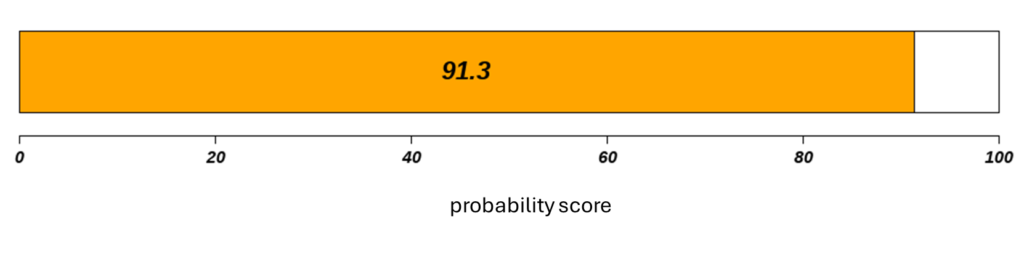
The report provided by Canhelp-UCa includes a probability score based on the analysis of the expression of specific genetic markers in urine, providing useful information for the early diagnosis and monitoring of bladder cancer recurrence. Thanks to the integration with artificial intelligence software, the report provides timely assessments, supporting clinical decisions and helping to optimize patient management.
If the probability score is:
- equal to or greater than 50: the result is to be considered positive
- less than 50: the result is to be considered negative
Bibliography
For more information click HERE
For information
Telephone
+39 0240090222
oncology@did.it
The content of this website is informative about the Quimark company and its products and services,
It is not intended as professional medical or health advice.