Canhelp®-Origin


Test Diagnostico per l’Identificazione dell’Origine del Tumore
Utilità di Canhelp-Origin
Canhelp-Origin è un test molecolare per l’identificazione del sito primario di un tumore metastatico,
particolarmente utile in caso di tumori scarsamente differenziati o non differenziati.
La tecnologia di Canhelp-Origin è RT-PCR quantitativa.
Il test è validato clinicamente, con un’accuratezza complessiva superiore al 90%.
E’ utile per il patologo molecolare, quando:
- Il tumore è scarsamente differenziato o indifferenziato
- Il campione è ridotto e ciò limita l’iter diagnostico
- I risultati dell’IHC sono inconclusivi o contraddicono i risultati precedenti
- È impossibile diagnosticare in modo differenziale due o più tipi di cancro
E’ utile per l’oncologo, quando:
- Il paziente ha una storia di tumori multipli
- si è in presenza di sedi metastatiche atipiche
- le diagnosi cliniche e patologiche sono incoerenti
- vi è un dubbio sulla diagnosi dopo un fallimento terapeutico
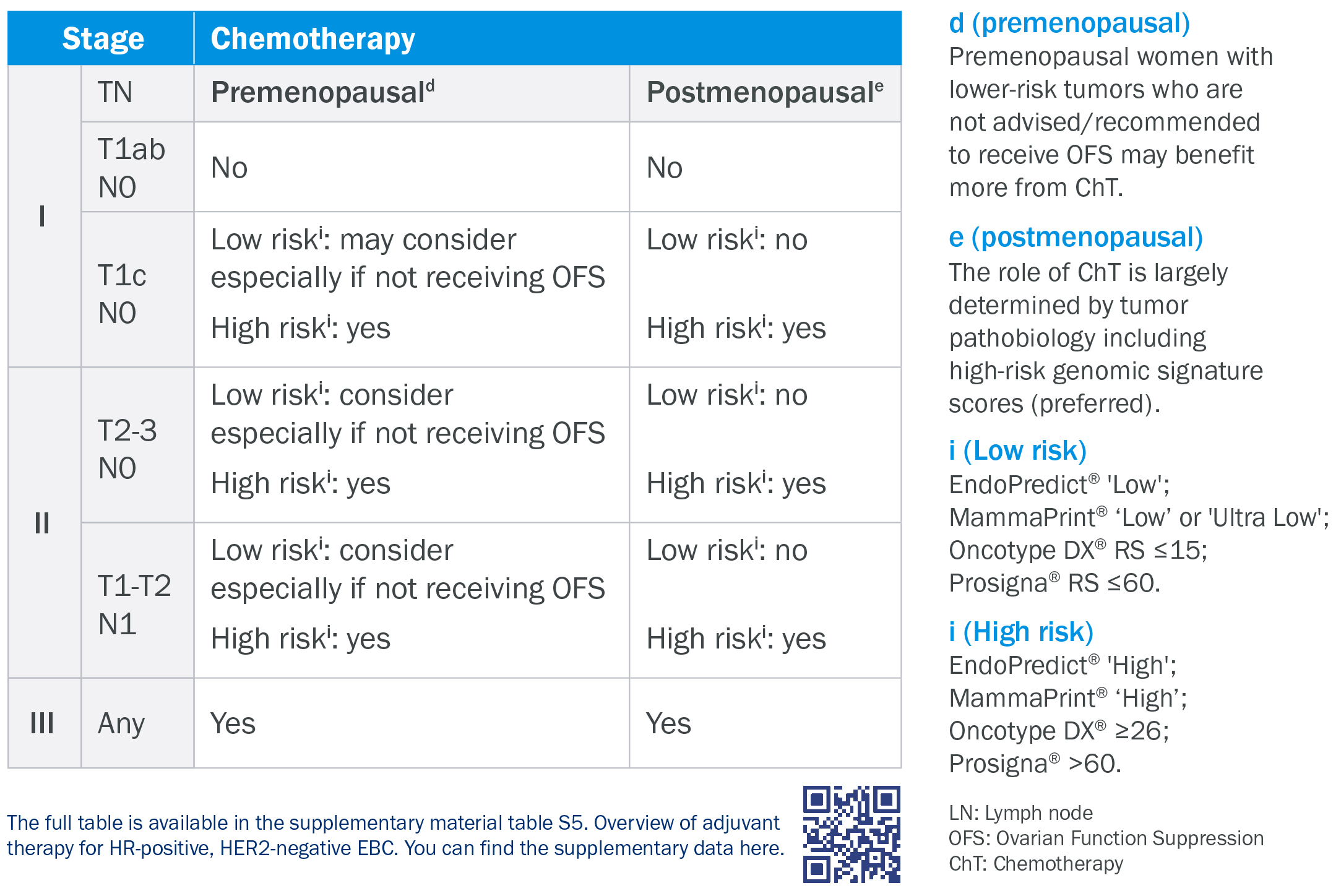
Level of evidence 1A (LoE1A)
The ESMO guidelines for early breast cancer recognize genomic tests such as EndoPredict at level 1A*.
* Loibl et al. Annals of Oncology, 2024 (v. supplement, table S5)
Prospective studies
Several prospective studies* with EndoPredict have been published, confirming the results of previous retrospective studies.
|
Publication
|
Key aspects |
|
Klein E. et al. Breast Cancer Res. Treat. 2024
|
|
* Penault-Llorca F. et al. ESMO Open 2024
* Klein E. et al. Breast Cancer Res. Treat. 2024
EndoPredict is a CE IVD kit
The EndoPredict test is performed on FFPE breast tumor tissue samples in your molecular laboratory.
Molecular analysis quantifies the expression of 12 genes, using messenger RNA extracted from formalin-fixed, paraffin-embedded (FFPE) material. The method uses RT-QPCR to investigate the expression of 12 genes:
• 5 genes linked to the expression of estrogen receptors: STC2, AZGP1, IL6ST, RBBP8, MGP
• 3 genes related to cell function (proliferation): BIRC5, UBE2C, DHCR7
• 4 control genes: OAZ1, CALM2, RPL37A, HBB
There are two kits available in different sizes that allow you to perform one or two tests at a time.
EndoPredict® QS UNO Kit – CE IVD (6 tests)
EndoPredict® QS DUO Kit – CE IVD (12 tests)

The total duration of the procedure is approximately 8 hours, of which 3 are operational, including the RNA extraction phase from the sample.
The EndoPredict report
The system uses the EPRG (EndoPredict Report Generator) interpretation and reporting software for the analysis of the 12 genes.
EPRG integrates the molecular data with information on the size of the tumor (T) and the number of positive lymph nodes (N) and generates the prognostic-predictive parameter EPclin which is the final result of the test.
The EndoPredict test report provides the following results:
- the risk of tumor recurrence at 10 years (prognostic data)
- the benefit of any chemotherapy (predictive data)
- the risk of recurrence up to 15 years (long-term prognostic data)
For information
Telephone
+39 0239261913
info@quimark.com
The content of this website is informative about the Quimark company and its products and services,
It is not intended as professional medical or health advice.